
Every year, around 700,000 people die by suicide worldwide (World Health Organisation, 2021). Many more make a suicide attempt, and even more struggle with ‘suicidal ideation’ – a broad term used to describe a range of contemplations, wishes, and preoccupations with death and suicide (Harmer et al., 2021). It is assumed that psychological disorders, particularly depression and bipolar disorder, contribute to most suicides. However, standard treatments for these disorders (both pharmacological and psychological) can take weeks to start working. This means that acute management of patients who are at high risk of suicide relies primarily on close monitoring and hospitalisation (NICE guidelines). Finding a treatment that could reduce suicidal ideation much more rapidly could therefore dramatically transform the management of acutely suicidal patients, relieving their distress and ultimately saving lives.
Several studies of ‘treatment-resistant depression’ (TRD) have shown that ketamine, which is primarily used as an anaesthetic, can have rapid antidepressant effects in sub-anaesthetic doses: it can reduce symptoms of depression within hours and this effect can last for up to a week (Kryst, 2020) (see previous Mental Elf blog). This discovery has opened a new avenue for TRD, and ketamine has increasingly been used off-label for TRD in specialised clinics (Wilkinson, 2017). Due to its rapid effect, an especially promising use of ketamine could be in reducing suicidal ideation.
Problematically, most clinical trials of ketamine have excluded patients who were at high risk of suicide. This is a common practice in antidepressant clinical trials, mainly because it is deemed unethical to administer a placebo to people at high risk of suicide, and because death by suicide could lead to termination of the trial. However, it means that the population that most needs anti-suicidal treatment is excluded from research. Several systematic reviews (Reinstatler, 2015; Bartoli, 2017; Wilkinson, 2017) have nonetheless pooled data from randomised controlled trials (RCTs) of ketamine that did include patients presenting with suicidal ideation at baseline and assessed ketamine’s effect on suicidal ideation as the primary outcome. They have concluded that ketamine shows promise for reducing suicidal ideation but noted that most of the assessed studies had small sample sizes and were not primarily designed to include patients with clinically significant levels of suicidal ideation, which limits the generalisability of the findings.
Since then, new trials – partially overcoming these limitations – have been published. Here, we appraise and summarise a recent systematic review by Hochschild, Grunebaum & Mann (2021), which sought to assess the strength of the evidence for ketamine as a rapid treatment for suicidal ideation when these new studies are considered.

If ketamine could reduce suicidal ideation within hours, it could dramatically transform the management of acutely suicidal patients – relieving their distress and ultimately saving lives.
Methods
The authors performed a comprehensive literature search and identified eleven RCTs comparing ketamine to placebo (saline) or active ingredient (midazolam – a benzodiazepine) that included patients presenting with clinically significant suicidal ideation and assessed the effect on suicidal ideation as a primary outcome.
The authors examined the effect of ketamine on suicidal ideation across these studies, including whether it was independent of ketamine’s antidepressant effect. They also assessed the effect of repeated doses and different ketamine preparations.
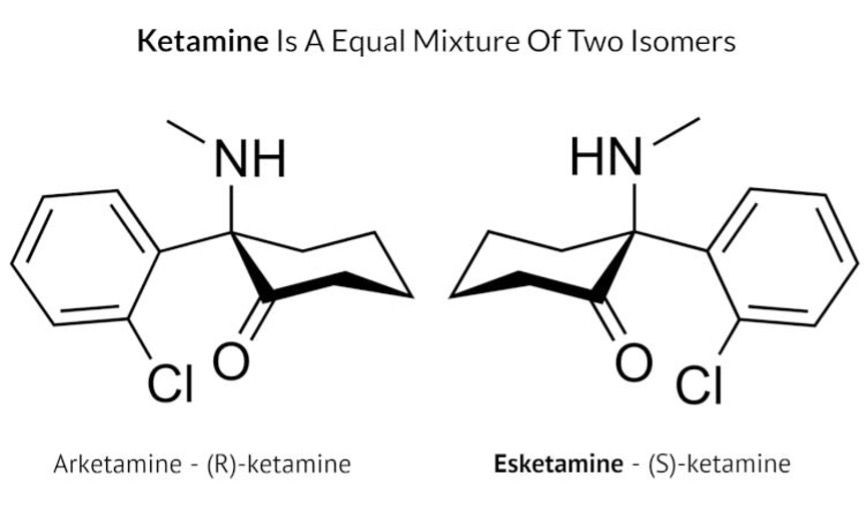
Ketamine is made up of two pairs of isomers, one is called (S)-ketamine and the other is called (R)-ketamine (see image below). Most studies included in this review used intravenous racemic ketamine, which contains both (S)-ketamine and (R)-ketamine in equal amounts, delivered directly into the bloodstream via intravenous infusion. Two studies used intranasal administration of esketamine, which contains just (S)-ketamine, sprayed into the nose. The authors did not conduct a meta-analysis on the results, so their study does not provide a statistical analysis of the effect of ketamine across the studies.
Results
Single dose of intravenous ketamine
- Four of the seven trials that examined a single dose of ketamine given intravenously found an anti-suicidal ideation effect at multiple, varying time points post-administration (Murrough et al., 2015; Fan et al., 2017; Grunebaum et al., 2018; Domany et al., 2020). However, this effect did not generally last beyond 72 hours (follow up for most of these studies was 7–14 days), although one study (Grunebaum et al., 2018) found that the effects of ketamine on suicidal ideation lasted for up to six weeks after initial infusion.
- Two of the studies (Grunebaum et al., 2018; Domany et al., 2020) reported that the effect of ketamine on suicidal ideation was at least partly independent from (or not fully explained by) its effect on depressive symptoms.
Multiple doses of intravenous ketamine
- Given that the reduction of suicidal ideation after a single dose of intravenous ketamine did not last beyond 72 hours post administration, an important question is whether the effect could be sustained by multiple doses. Here, the evidence is inconclusive.
- The authors identified only one randomised double-blind trial assessing the effect of multiple infusions of ketamine (Ionescu et al., 2019): in this trial, the effects of repeated infusions were not superior to multiple infusions of saline placebo.
- Another study reviewed by the authors (Phillips et al., 2020) did find a superior effect of repeated infusions over placebo, but this was assessed in the open-label cross-over phase (in which patients who initially did not respond to placebo received six ketamine infusions, and patients who responded to ketamine received four additional ketamine infusions), not in the randomised double-blind phase (which compared a single infusion of ketamine to placebo) and should therefore be interpreted only tentatively.
Nasal administration of esketamine
- The authors identified two studies investigating esketamine nasal spray, but neither study found it to be superior to placebo. The authors suggested that this could be due to lower bioavailability and greater severity of side effects of esketamine following nasal administration.
Some studies suggested that intravenously administered esketamine reduced suicidal ideation compared with midazolam/placebo. Image copyright © 2019 Pharmacist Answers
Conclusions
The authors concluded that:
ketamine shows promise as an antidepressant and specific anti-SI [suicidal ideation] agent. Replication of research findings supporting its use are needed in larger scale trials, in diverse clinical settings, and with alternative dosing and administration methods.

We have reported on ketamine as a “promising” new treatment for depression and suicidal ideation for some years now. Will this promise ever be realised?
Strengths and limitations
Strengths
- This study is the most up-to-date systematic review of ketamine’s effect on suicidal ideation and included eleven RCTs of ketamine.
- Most of the trials were judged to have a low risk of bias according to the Revised Cochrane risk-of-bias tool for randomised trials, which assesses potential bias across five domains: randomisation process, digression from planned interventions, missing data, outcome measurement, and choice of reported results.
- The conclusions of this study generally agree with three additional (partially overlapping) systematic reviews on this topic which were published at approximately the same time (Witt et al., 2020; Siegel et al., 2020; Wilkinson et al., 2018), which increases confidence in the discussed conclusions. Furthermore, Witt et al., (2020) performed a meta-analysis within their systematic review; pooling data from various studies over five different time points and providing an estimate of effect size. They demonstrated that the effect is significant for all time-points up to 72 hours, but not significant thereafter. Hence, these meta-analytic results are in line with the present review.
Limitations
- As this study did not perform a meta-analysis, it does not provide a robust summary estimate of ketamine’s effectiveness, but nonetheless offers preliminary evidence of ketamine’s potential effect on reducing suicidal ideation. These findings are consistent with those of previous a meta-analysis conducted by Wilkinson et al. (2018), although these authors also included studies that were not specifically designed to investigate outcomes in patients with baseline suicidal ideation.
- A total sample of 611 patients was included in this review. Although the sample sizes of the included esketamine trials were relatively large (mean = 147), the sizes of the included ketamine trials were relatively low (mean = 31.7), with five trials including under 30 participants.
- There was considerable heterogeneity in dosing and route of administration in the studies assessed by this review, which somewhat limits comparability across studies. Further large RCTs are needed to establish what is the most effective dose and route of administration.
- Blinding efficacy was not reported in this systematic review. Furthermore, although the use of a placebo (e.g., saline) or active control (e.g., midazolam) was reported in a table within the review, the authors did not provide a direct comparison between trials that used placebo versus those that used an active control.
- Many studies included in this review relied on assessing ketamine’s effect on suicidal ideation using changes on a single item on a depression rating scale. However, this may not be a sufficiently sensitive measure for this purpose, especially when assessing rapid changes in suicidal ideation over a short time-period (Witt et al., 2020).

There was considerable heterogeneity between studies included in this review and the authors did not conduct a meta-analysis.
Implications for practice
Despite these promising findings, several important questions need to be addressed before ketamine can be regarded as safe and effective for use in a clinical setting as a treatment for patients at risk for suicide.
- Even if the effect of ketamine on reducing suicidal ideation is confirmed in larger studies, it is an open question whether this will also lead to a reduction in suicidal behaviour. Suicidal ideation is a poor predictor of suicidal attempts and completed suicide in people with depression (Rogers et al., 2018), and the effect on suicidal ideation may not necessarily translate to reduced suicidal behaviour. There are no rigorous trials investigating the effect of ketamine on suicidal attempts or completed suicides, as this would require much larger samples (because suicides are relatively rare) and would present considerable ethical and practical challenges (Perlis et al., 2015).
- Long-term use of (high doses of) ketamine is known to cause damage to the brain and urinary system and carries a non-negligible potential for abuse. There are also potential adverse effects (e.g., dissociation, blurred vision, dizziness, malaise, nausea, or possible emergence psychosis) that can occur beyond the few hours after treatment (Short et al., 2018). The safety profile of ketamine as an anti-suicidal ideation agent, especially if used in repeated doses, remains to be established.
- The effect of ketamine on reducing suicidal ideation appears to be relatively short-lived (only up to 72 hours post-infusion) and it is uncertain whether it can be sustained by repeated administrations. There is also the possibility of rebound suicidal ideation, possibly leading to an increased risk of suicide after the initial effects wear off. To address these issues, future studies would benefit from longer follow-ups, especially if ketamine were to be used for response maintenance and relapse prevention.
- Almost all trials of ketamine have been performed in patients with mood disorders (depression or bipolar disorder). It is to be established whether the results will generalise to suicidal ideation outside the context of mood disorders. Given ketamine’s psychotomimetic effects and potential for abuse, it may not be a suitable agent for patients with psychotic and substance use disorders and such patients have been excluded from virtually all ketamine trials.

A great deal of uncertainty remains about ketamine as a safe and effective treatment for mental illness.
Statement of interests
None.
Links
Primary paper
Other references
Photo credits
- Photo by Zach Vessels on Unsplash
- Photo by Sinitta Leunen on Unsplash
- Photo by Jametlene Reskp on Unsplash
