Deliberate self-harm amongst young people is a rapidly growing phenomena with as many as 45% of adolescents thought to have tried it at some point (Nock, 2010; Muehlenkamp et al., 2012). For the most part, self-harm is a form of non-suicidal self-injury (NSSI), that is, a deliberate act of harm without the intention of dying (Nock 2010; Brent et al. 2013). Whilst self-harm may not start with suicidal intentions, it is recognised to be a significant risk factor for future attempts (Asarnow et al. 2011; Chesin et al. 2017). Understanding more about connections in the parts of the brain active or underactive in self-harm, may help in developing targeted therapies thus preventing decline into suicidal behaviours. It may also help challenge the stigma around NSSI being a form of attention-seeking behaviour (Burke et al. 2019; Favazza, 1998; Gratz, 2006).
It has long been understood that NSSI is a dysfunctional coping strategy to help regulate emotional distress (Klonsky et al. 2011; Nock 2010). But why and how does the act of doing something that seemingly goes against nature, help with emotion regulation? The answer lies within activation of the amygdala (Plener et al. 2012), the brain’s emotion control centre; physiological research demonstrates high levels of activity in this region in young people who self-harm (Nock & Mendes 2008).
Research using functional magnetic resonance imaging (fMRI) has revealed poor connections between the amygdala and frontal areas of the brain such as the medial prefrontal cortex (mPFC) (Banks et al., 2007), the part of the brain that underpins higher level thinking (Wood & Grafman, 2003) and crucial to decision making and impulsivity (Bechara et al. 1994; Ingvar, 1994; Domsalla et al. 2014). Research has shown psychological interventions to be effective at improving connectivity between the amygdala and mPFC (Hölzel et al., 2009; Ritchey et al., 2011) which in turn has been effective in preventing NSSI (Ougrin et al., 2015).

It has previously been demonstrated that people who self-harm have an over-active amygdala (emotional centre of the brain) and poor connectivity with between the amygdala and medial prefrontal cortex (responsible for higher order thinking).
Methods
This research hoped to build on previous work by using Functional magnetic resonance imaging (fMRI) to examine the brains of adolescents who self- harm in order to:
- determine how connectivity of the amygdala and frontal regions of the brain differs when compared to healthy controls (HCs);
- establish how connectivity patterns alter in response to psychological interventions.
In order to achieve this, the resting-state functional connectivity (RsFC) between the amygdala, the mPFC and other regions of interest were examined.
It was hypothesised that:
- Adolescents who self-harmed would show impaired amygdala-mPFC connectivity when compared to HCs.
- Psychological intervention would result in better functional connectivity
24 patients, aged 12 to 18, who repeatedly self-harmed but did not have another comorbid mental health disorder, agreed to take part in the study with consent from at least 1 parent or guardian also willing to engage in the psychological treatment. Patients were all chosen from the outpatient clinic of the Department of Child and Adolescent Psychiatry and Psychology in Barcelona.
17 healthy control subjects (HCs) were recruited from adverts in the community and school and were matched as far as possible in age, gender and socio economic status, with the patients. Patients and HCs then went through the following process:
Scans examined the RsFC between:
- The amygdala and the cluster of the brain that includes the anterior cingulate cortex (ACC), the subcallosal cortex and the paracingulate gyrus.
- The amygdala and a cluster of the brain that includes the right planum temporale, and the right insula.
- The mPFC and the left insula.
- The mPFC and the precentral and postcentral gyrus.
All 24 patients took part in 16 weeks of psychological therapy commonly used in the author’s outpatient clinics, either Dialectical Behaviour Therapy for adolescents (DBT-A) or Treatment-as-Usual (TAU) with additional group sessions.
Results
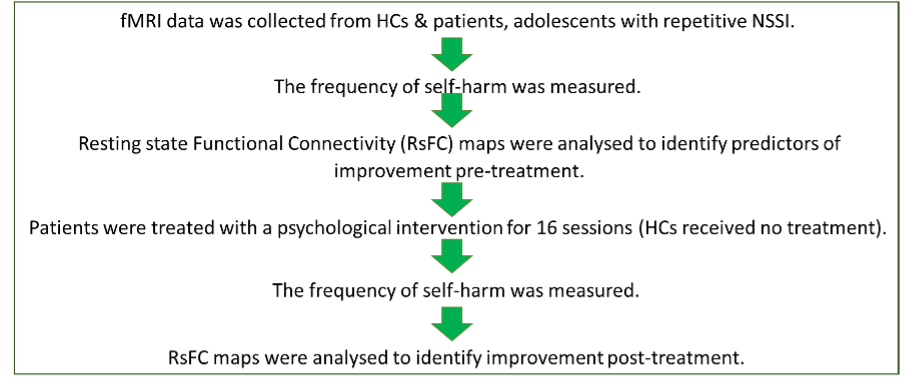
Significant differences were found between patients and health controls in all areas of RsFC:
All 24 patients had a lower RsFC than the HCs suggesting that this is a contributory factor for self-harm, thereby increasing understanding of why self-harm is used and challenging stigma around this. After treatment 12 patients (50%) reported a reduction in the amount of times they self-harmed, 10 patients (41.6%) reported no change and 2 patients (8.3%) said that their level of self-harm had increased.
For the areas of the brain specifically examined the results reveal:
1. The amygdala and the ACC, the subcallosal cortex and the paracingulate gyrus.
Patients with NSSI had decreased connectivity within this area. As the ACC is important in emotion and mood regulation, this may explain why adolescents with NSSI are often hyper sensitive to emotional stimuli (Plener et al., 2012) which can lead to depression (Connolly et al., 2017) or suicidal behaviour (Cox Lippard et al., 2014; Johnston et al., 2017).
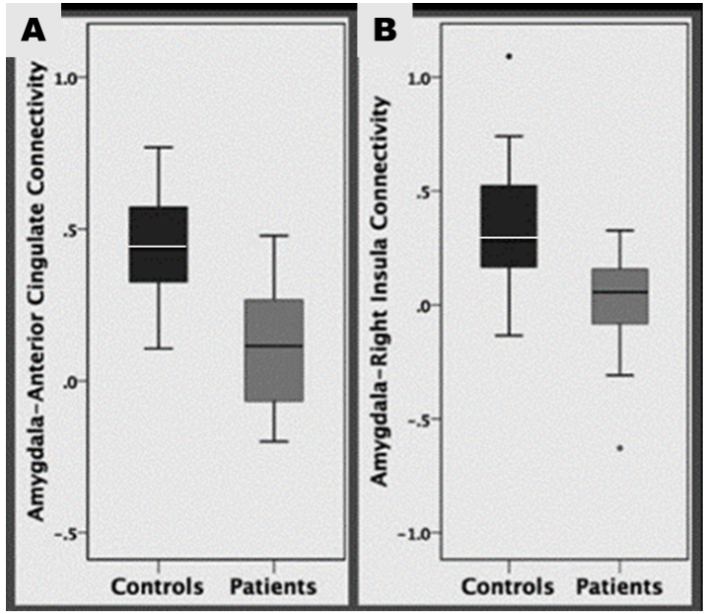
2. The amygdala and the right planum temporale, and the right insula.
Connectivity between the amygdala and insula was poor amongst patients. This connection plays a key role in transmitting information about internal states including pain perception (Westlund Schreiner et al., 2015). It has been suggested that patients who self-harm, have altered pain perception (Ballard et al., 2010), something supported by other studies (Bonenberger et al., 2015).
3. The mPFC and the left insula.
The study also revealed decreased mPFC left insula connectivity, implicated in pain processing (Zhang et al., 2014), which may contribute to impaired pain perception in self harming adolescents. As this area of the brain is also linked to social cognition and empathy, it is possible that the ability to recognise the impact such behaviour has on others may also be impaired. If this can be proven, it is important to our understanding as those who engage in self-harm can be perceived as selfish or manipulative.
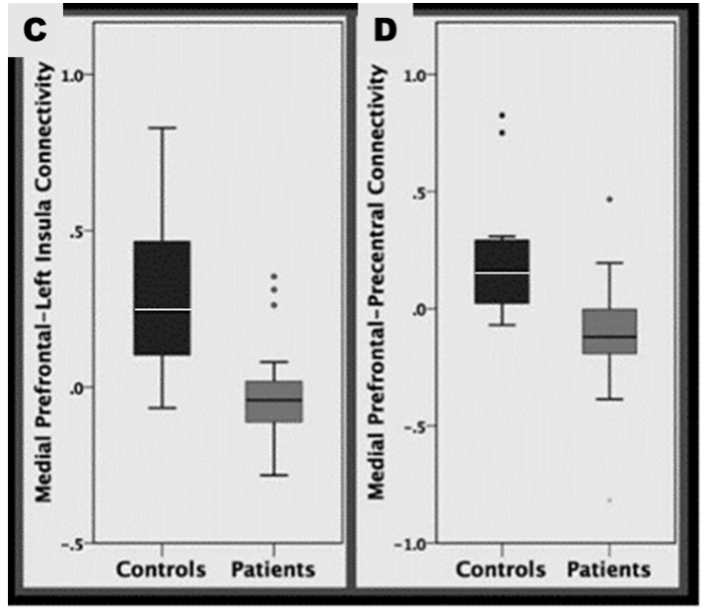
4. The mPFC and the precentral-postcentral gyrus.
Poor connectivity between the mPFC and precentral-postcentral gyri, with regards to NSSI, is something that had not been reported on at the time this study took place. The precentral gyrus is responsible for motor function linked to voluntary movement (Exner et al., 2002), cognitive processing and emotion regulation (Li et al., 2017). The decreased connectivity could allow the person to use self-harm to regulate their emotional responses and reduce negative emotions. The postcentral gyrus is the key receptor of general bodily sensation (Webb, 2017), decreased connectivity may be reflective of atypical processing of sensory information. Further studies are required to confirm these interpretations.
The second objective of the study was to identify whether connectivity patterns at baseline would predict a positive response to psychological intervention self-harm. The study revealed that reduced mPFC-amygdala connectivity at baseline was associated with greater improvement in NSSI at the end of treatment, something confirmed by other studies (Groschwitz & Plener, 2012; Niedtfeld et al., 2010; Plener et al., 2012; Schreiner et al., 2017).
The results from this study suggests that psychotherapy may enhance skills associated with mPFC thereby increasing the ability to regulate emotions. The 12 patients who reported a reduction in their levels of NSSI showed improved connectivity between their mPFC and other parts of the brain.

Adolescents who self-harmed had lower connectivity between the amygdala and medial prefrontal cortex. An improvement in connection after therapy correlated with a reduction in NSSI.
Conclusions
This study, whilst small, increases our understanding of how brain network connectivity can present for young people with NSSI. This is significant, not only because it may help predict patients that would better respond to psychotherapy, but also in challenging the stigma attached to young people who self-harm. If we can demonstrate a neurological difference, perceptions around seeking help and getting treatment may change.

Understanding that brain network connectivity is different in young people who self-harm could help challenge the stigma attached.
Strengths and limitations
The size of the sample was small which limits the generalisability of this study, larger studies would be needed to confirm the findings.
Due to the small size of the sample adherence to the DBT-A programme was not required but it is unlikely that in a larger study the same levels of fidelity would be shown and this is something that would need to be monitored. The study was also not clear about any differences relating to DBT-A and TAU.
Implications for practice
Proving that poor connectivity between the amygdala and mPFC is an indicator of NSSI is significant as it:
- Helps remove the guilt, shame and stigma associated with self-harm;
- Reveals who is likely to respond to lengthy psychotherapy;
- Offers opportunities for further research into the most effective forms of therapy.

Demonstrating aberrant brain connectivity is involved in NSSI could help remove the patient’s guilt and shame.
Conflicts of interest
Dr Singh and Dr Sugranyes (researchers who worked on this paper) both received research funding from the Brain and Behaviour Foundation with additional funding received from Johnsons and Johnson, Otsuka Pharmaceuticals, Jansen, and Adamed Farma.
The blogger Rachel Symons has no conflicts of interest.
Links
Primary paper
Santamarina-Perez, P., Romero, S., Mendez, I., Leslie, S. M., Packer, M. M., Sugranyes, G., Picado, M., Font, E., Moreno, E., Martinez, E., Morer, A., Romero, M., & Singh, M. K. (2019). Fronto-Limbic Connectivity as a Predictor of Improvement in Nonsuicidal Self-Injury in Adolescents Following Psychotherapy. Journal of child and adolescent psychopharmacology, 29(6), 456–465. https://doi.org/10.1089/cap.2018.0152
Other references
Asarnow, J. R., Porta, G., Spirito, A., Emslie, G., Clarke, G., Wagner, K. D., Vitiello, B., Keller, M.,
Ballard, E., Bosk, A. & Pao, M. (2010). Invited Commentary: Understanding Brain Mechanisms of Pain Processing in Adolescents’ Non-Suicidal Self-Injury. Journal of Youth and Adolescence, 39, 327–334. https://doi.org/10.1007/s10964-009-9457-1
Banks, S. J., Eddy, K. T., Angstadt, M., Nathan, P. J., & Phan, K. L. (2007). Amygdala-frontal connectivity during emotion regulation. Social cognitive and affective neuroscience, 2(4), 303–312. https://doi.org/10.1093/scan/nsm029
Bechara, A., Damasio, A. R., Damasio, H., & Anderson, S. W. (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 50(1-3), 7–15. https://doi.org/10.1016/0010-0277(94)90018-3
Birmaher, B., McCracken, J., Mayes, T., Berk, M., & Brent, D. A. (2011). Suicide attempts and nonsuicidal self-injury in the treatment of resistant depression in adolescents: findings from the TORDIA study. Journal of the American Academy of Child and Adolescent Psychiatry, 50(8), 772–781. https://doi.org/10.1016/j.jaac.2011.04.003
Bonenberger, M., Plener, P. L., Groschwitz, R. C., Grön, G., & Abler, B. (2015). Differential neural processing of unpleasant haptic sensations in somatic and affective partitions of the insula in non-suicidal self-injury (NSSI). Psychiatry research, 234(3), 298–304. https://doi.org/10.1016/j.pscychresns.2015.10.013
Brent, D. A., McMakin, D. L., Kennard, B. D., Goldstein, T. R., Mayes, T. L., & Douaihy, A. B. (2013). Protecting adolescents from self-harm: a critical review of intervention studies. Journal of the American Academy of Child and Adolescent Psychiatry, 52(12), 1260–1271. https://doi.org/10.1016/j.jaac.2013.09.009
Burke, T. A., Piccirillo, M. L., Moore-Berg, S. L., Alloy, L. B., & Heimberg, R. G. (2019). The stigmatization of nonsuicidal self-injury. Journal of clinical psychology, 75(3), 481–498. https://doi.org/10.1002/jclp.22713
Chesin, M. S., Galfavy, H., Sonmez, C. C., Wong, A., Oquendo, M. A., Mann, J. J., & Stanley, B. (2017). Nonsuicidal Self-Injury Is Predictive of Suicide Attempts Among Individuals with Mood Disorders. Suicide & life-threatening behavior, 47(5), 567–579. https://doi.org/10.1111/sltb.12331
Connolly, C. G., Ho, T. C., Blom, E. H., LeWinn, K. Z., Sacchet, M. D., Tymofiyeva, O., Simmons, A. N., & Yang, T. T. (2017). Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. Journal of affective disorders, 207, 86–94. https://doi.org/10.1016/j.jad.2016.09.026
Cox Lippard, E. T., Johnston, J. A., & Blumberg, H. P. (2014). Neurobiological risk factors for suicide: insights from brain imaging. American journal of preventive medicine, 47(3 Suppl 2), S152–S162. https://doi.org/10.1016/j.amepre.2014.06.009
Domsalla, M., Koppe, G., Niedtfeld, I., Vollstädt-Klein, S., Schmahl, C., Bohus, M., & Lis, S. (2014). Cerebral processing of social rejection in patients with borderline personality disorder. Social cognitive and affective neuroscience, 9(11), 1789–1797. https://doi.org/10.1093/scan/nst176
Exner, C., Koschack, J., & Irle, E. (2002). The differential role of premotor frontal cortex and basal ganglia in motor sequence learning: evidence from focal basal ganglia lesions. Learning & memory (Cold Spring Harbor, N.Y.), 9(6), 376–386. https://doi.org/10.1101/lm.48402
Favazza, A. R. (1998). The coming of age of self-mutilation. The Journal of nervous and mental disease, 186(5), 259–268. https://doi.org/10.1097/00005053-199805000-00001
Gratz, K. L. (2006). Risk factors for and functions of deliberate self-harm: An empirical and conceptual review. Clinical Psychology: Science and Practice, 10, 192–205. 10.1093/clipsy.bpg022
Groschwitz, R. C., & Plener, P. L. (2012). The Neurobiology of Non-suicidal Self-injury (NSSI): A review. Suicidology Online, 3, 24-32.
Hölzel, B. K., Carmody, J., Evans, K. C., Hoge, E. A., Dusek, J. A., Morgan, L., Pitman, R. K., & Lazar, S. W. (2010). Stress reduction correlates with structural changes in the amygdala. Social cognitive and affective neuroscience, 5(1), 11–17. https://doi.org/10.1093/scan/nsp034
Ingvar D. H. (1994). The will of the brain: cerebral correlates of willful acts. Journal of theoretical biology, 171(1), 7–12. https://doi.org/10.1006/jtbi.1994.1206
Johnston, J., Wang, F., Liu, J., Blond, B. N., Wallace, A., Liu, J., Spencer, L., Cox Lippard, E. T., Purves, K. L., Landeros-Weisenberger, A., Hermes, E., Pittman, B., Zhang, S., King, R., Martin, A., Oquendo, M. A., & Blumberg, H. P. (2017). Multimodal Neuroimaging of Frontolimbic Structure and Function Associated With Suicide Attempts in Adolescents and Young Adults With Bipolar Disorder. The American journal of psychiatry, 174(7), 667–675. https://doi.org/10.1176/appi.ajp.2016.15050652
Klonsky, D. E., Muehlenkamp, J. J., Lewis, S. P., & Walsh, B. (2011). Nonsuicidal Self-Injury. Boston, USA: Hogrefe Publishing Corp.
Li, J., Wang, Z., Hwang, J., Zhao, B., Yang, X., Xin, S., Wang, Y., et al. (2017). Anatomical brain difference of subthreshold depression in young and middle-aged individuals. NeuroImage : Clinical 14(1): 546-551. http://dx.doi.org/10.1016/j.nicl.2017.02.022.
Muehlenkamp, J. J., Claes, L., Havertape, L., & Plenner, L. P. (2012). International prevalence of adolescent non-suicidal self-injury and deliberate self-harm. Child and Adolescent Psychiatry and Mental Health 6(10), doi.org/10.1186/1753-2000-6-10.
Niedtfeld, I., Schulze, L., Kirsch, P., Herpertz, S. C., Bohus, M., & Schmahl, C. (2010). Affect regulation and pain in borderline personality disorder: a possible link to the understanding of self-injury. Biological psychiatry, 68(4), 383–391. https://doi.org/10.1016/j.biopsych.2010.04.015
Nock, M. K. (2010). Self-injury. Annual Review of Clinical Psychology, 6, 339–63. doi.org/10.1146/annurev.clinpsy.121208.131258
Nock, M. K., & Mendes, W. B. (2008). Physiological arousal, distress tolerance, and social problem-solving deficits among adolescent self-injurers. Journal of consulting and clinical psychology, 76(1), 28–38. https://doi.org/10.1037/0022-006X.76.1.28
Ougrin, D., Tranah, T., Stahl, D., Moran, P., & Asarnow, J. R. (2015). Therapeutic interventions for suicide attempts and self-harm in adolescents: systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 54(2), 97–107.e2. https://doi.org/10.1016/j.jaac.2014.10.009
Plener, P. L., Bubalo, N., Fladung, A. K., Ludolph, A. G., & Lulé, D. (2012). Prone to excitement: adolescent females with Non-suicidal self-injury (NSSI) show altered cortical pattern to emotional and NSS-related material. Psychiatry research, 203(2-3), 146–152. https://doi.org/10.1016/j.pscychresns.2011.12.012
Ritchey, M., Dolcos, F., Eddington, K. M., Strauman, T. J., & Cabeza, R. (2011). Neural correlates of emotional processing in depression: changes with cognitive behavioral therapy and predictors of treatment response. Journal of psychiatric research, 45(5), 577–587. https://doi.org/10.1016/j.jpsychires.2010.09.007
Webb, W. G. (2017) Organisation of the nervous system I. In: Neurology for the Speech-Language Pathologist. Edited by Webb, W., & Adler, R. K. St Louis, USA: Elsevier. Pp 13-43.
Westlund Schreiner, M., Klimes-Dougan, B., Begnel, E. D., & Cullen, K. R. (2015). Conceptualizing the neurobiology of non-suicidal self-injury from the perspective of the Research Domain Criteria Project. Neuroscience and biobehavioral reviews, 57, 381–391. https://doi.org/10.1016/j.neubiorev.2015.09.011
Westlund Schreiner, M., Klimes-Dougan, B., Mueller, B. A., Eberly, L. E., Reigstad, K. M., Carstedt, P. A., Thomas, K. M., Hunt, R. H., Lim, K. O., & Cullen, K. R. (2017). Multi-modal neuroimaging of adolescents with non-suicidal self-injury: Amygdala functional connectivity. Journal of affective disorders, 221, 47–55. https://doi.org/10.1016/j.jad.2017.06.004
Wood, J. N., & Grafman, J. (2003). Human prefrontal cortex: processing and representational perspectives. Nature Reviews Neuroscience. 4, 139–147. doi: 10.1038/nrn1033
Zhang, S., Wu, W., Huang, G., Liu, Z., Guo, S., Yang, J., & Wang, K. (2014). Resting-state connectivity in the default mode network and insula during experimental low back pain. Neural regeneration research, 9(2), 135–142. https://doi.org/10.4103/1673-5374.125341
Photo credits
- Photo by Jamie Street on Unsplash
- Photo by Joana Abreu on Unsplash
- Photo by Brett Jordan on Unsplash