Nonsteroidal anti-inflammatories (NSAIDS) such as ibuprofen are commonly prescribed following third molar surgery. Ibuprofen inhibits both cyclooxygenase isoforms 1 and 2 (Cox-1 and Cox-2). Cox-2 inhibitors avoid the gastrointestinal problems associated with Cox-1 inhibition.
This systematic review and meta-analysis set out to compare Cox-2 inhibitors with ibuprofen in terms of analgesia, rescue medication use, and adverse effects following third molar surgery.(González-Barnadas et al., 2019)
Methods
The primary outcome measure was Pain relief 6, 8, and 12 h after third molar extraction:
the outcome measure of total pain relief (TOTPAR) was obtained by dichotomizing the data to achieve a proportion of patients achieving 50% pain relief (50% maximum TOTPAR).
The secondary outcome measures were:
- Rescue analgesia (the proportion of patients who needed rescue medication 24 h after third molar extraction).
- Adverse effects (the proportion of patients experiencing any adverse effect during the entire postoperative follow-up period).
Two authors searched the following databases: MEDLINE, Scopus, Web of Science, International Pharmaceutical Abstracts, and The Cochrane Library databases up to 9 November 2017 was conducted to identify all relevant human randomised control trails (RCTs) without year or language restrictions. Grey literature was searched on Clinicaltrials.gov, OpenGrey , and the International Clinical Trial Registry Platform.
The search terms were (((((((((Cyclooxygenase 2 Inhibitors [Mesh]) OR meloxicam) OR rofecoxib) OR
lumiracoxib) OR etoricoxib) OR celecoxib) OR valdecoxib) OR parecoxib) OR coxibs) AND (((((third molar [Mesh]) OR third molar) OR wisdom tooth) OR wisdom teeth) OR third molar surgery [Mesh]).
Risk of bias (RoB) was assessed using the Cochrane Collaboration’s tool for assessing risk of bias.
Results
- 12 studies were included for the qualitative analysis and eight RCT’s for the meta-analysis.
- The RoB assessment considered 10/12 studies at uncertain risk of bias and 2/12 at high risk.
- There were no significant differences in the summary estimate between drugs at 6, 8, and 12 hours (Table 1).
| TOTPAR after (hours) | Summary estimate(OR) | 95% Confidence interval | p-value |
| 6 | 0.95 | 0.65 to 1.39 | 0.78 |
| 8 | 1.20 | 0.84 to 1.72 | 0.31 |
| 12 | 1.48 | 0.88 to 2.49 | 0.14 |
Table 1. Summary estimates for metanalysis of Total Pain Relief (TOPAR)
- The need for rescue analgesia after 24 hours significantly favoured ibuprofen OR=0.3 (95% CI: 0.2 to 0.46) p=<0.001.
- Meta-analysis of adverse events indicated that ibuprofen intake was related to nausea (OR = 0.57, 95%CI: 0.38 to 0.86, p = 0.007, I2 = 0%) and vomiting (OR = 0.50, 95%CI 0.29–0.87, p = 0.01, I2 = 0%). No statistically significant differences were found for headache, dizziness, or alveolar osteitis
Conclusions
The authors concluded: –
Selective COX-2 inhibitors and ibuprofen afford similar pain relief 6, 8, and 12 h after impacted third molar extraction, Lumiracoxib 400 mg shows significantly better results than ibuprofen 400 mg after 12 h, while celecoxib 200 mg performs significantly worse 8 h after extraction. In single-dose studies, patients receiving ibuprofen require more analgesic rescue medication after 24 h. Also, there is a tendency to obtain better results with selective COX-2 inhibitors when the postoperative time increases, probably due to the longer duration of their effect. The incidence of one or more adverse events is comparable between the two groups. However, ibuprofen intake is related to an increase probability of developing nausea and vomiting.
Comments
This was a well-constructed systematic review (SR) producing a logical answer to both their primary and secondary research questions despite the large amount of heterogeneity between the studies. There are a few important points that could have improved the quality of this SR:
- The methodology would have been improved by preregistering the protocol on PROSPERO especially in light of using the PRISMA guidelines (Shamseer et al., 2015).
- In their conclusion the authors incorrectly state that ‘Lumiracoxib 400 mg shows significantly better results than ibuprofen 400 mg’ as this was only in a single underpowered study involving 75 participants. To support their conclusion the study would require double the number of participants to be correctly powered (1-β = 0.8).
- The authors converted the continuous data into dichotomous using the following justification:
“According to McQuay (McQuay et al., 1996), the summary values of pain relief in clinical trials on analgesics following standard trial methods exhibit a non-normal distribution; as a result, using mean values to describe these summary values is inappropriate and may lead to erroneous conclusions. For this reason, in the present study, continuous data were converted into dichotomous data in order to avoid such errors.”
Having read the McQuay paper the problem related to the non-linear distribution of pain relief in the placebo arm of the pain relief trial. The authors of this SR were not comparing the Cox-2 inhibiter with placebo so they could have undertaken the analysis on the continuous data rather than convert it to dichotomous data and odds ratios (OR).
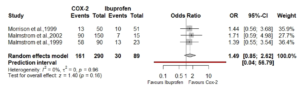
- For the reason stated above the meta-analysis should have been carried out using the untransformed continuous data. I have attached a forest plot showing a section of the authors estimate as an OR (Fig.1)
Figure 1. Forest plot at 8 hrs post-op using published data.
I have taken the raw data the authors extracted for this subgroup from Table 2. and created a new meta-analysis and forest plot using standardised mean difference (Cohens d), and a random effects model with a Hartung-Knapp adjustment for small study numbers. (Fig 2.)
Figure 2, Meta-analysis of the same data (red box above) using standardised mean difference (Cohen’s d)
From this new analysis it is still possible to derive the same conclusions without overcomplicating data extraction and synthesis. In addition, the wide confidence interval surrounding the prediction interval confirms the wide variability to be expected in future studies.
Links
Primary Paper
González-Barnadas, A., Camps-Font, O., Martín-Fatás, P., Figueiredo, R., Gay-Escoda, C. and Valmaseda-Castellón, E. (2019) ‘Efficacy and safety of selective COX-2 inhibitors for pain management after third molar removal: a meta-analysis of randomized clinical trials’, Clinical Oral Investigations. Clinical Oral Investigations. doi:10.1007/s00784-019-02910-3.
Other References
McQuay, H., Carroll, D. and Moore, A. (1996) ‘Variation in the placebo effect in randomised controlled trials of analgesics: All is as blind as it seems’, Pain, 64(2), pp. 331–335. doi: 10.1016/0304-3959(95)00116-6.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015 Jan 2;350:g7647. doi: 10.1136/bmj.g7647. Erratum in: BMJ. 2016 Jul 21;354:i4086. PubMed PMID: 25555855
Dental Elf 21st Jan 2014