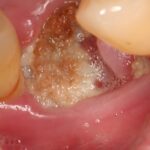
Medication-related osteonecrosis of the jaw (MRONJ) was first reported in 2003 associated with bisphosphonate treatment/ It has since been reported in patients taking denosumab and anti-angiogenic agents. MRONJ is a severe adverse reaction seen in some patients that results in progressive destruction of bone in the mandible or maxilla. The exact mechanisms underlying MRONJ are unknown and its frequency ranges from very rare to common with the type of drug, dosage, treatment indication and duration.
The aim of this Cochrane review update was to assess interventions for the prevention or treatment of osteonecrosis of the jaw compared with each other or compared with no treatment or an inactive intervention
Methods
Searches were conducted in the Cochrane Oral Health’s Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) Medline, Embase, the US National Institutes of Health Ongoing Trials Register and the World Health Organization International Clinical Trials Registry Platform without restriction on date or language. Randomised controlled trials (RCTs) comparing one modality of intervention with another for the prevention or treatment of MRONJ were considered. Study selection, data abstraction, and risk of assessment bias was carried out independently by two reviewers. For MRONJ prevention the primary outcome was the incidence of MRONJ. For MORONJ treatment the primary outcome was healing of MRONJ. Dichotomous outcomes were reported as risk ratio (RR) with 95% confidence intervals (CI). Meta-analysis was not conducted because of the range of different interventions examined.
Results
- 13 RCTs involving 1668 patients were included
- 5 RCTs considered prevention.
- 1RCT in men with metastatic prostate cancer treated with zoledronic acid. Compared standard care with 3 monthly examinations and prevention showing a lower MRONJ risk, RR= 0.10 (95%CI; 0.02 to 0.39).
- 5 RCTs tested various methods to risk of postoperative MRONJ after dentoalveolar surgery
- Plasma rich in growth factors inserted into the post extraction alveolus in addition to standardised medical and surgical care versus standardised medical and surgical care alone RR = 0.08 (95%CI; 0.00 to 1.51) [176 patients].
- Delicate surgery and closure by primary intention versus non-traumatic tooth avulsion and closure by secondary intention (no postoperative MRONJ in either group)
- Primary closure of the extraction socket with a mucoperiosteal flap versus application of platelet-rich fibrin without primary wound closure intention (no postoperative MRONJ in either group)
- Subperiosteal wound closure versus epiperiosteal wound closure RR = 0.09 (95%CI; 0.00 to 1.56) [132 patients].
- 8 RCTs considered different treatment options for MRONJ
- One RCT (46 patients) compared hyperbaric oxygen (HBO) treatment plus standard care (antiseptic rinses, antibiotics, and surgery) with standard care alone RR = 1.56 (95%CI; 0.77 to 3.18).
- One study found no difference in MRONJ healing rates was seen between autofluorescence-guided bone surgery and conventional bone surgery RR = 1.08 (95%CI 0.85 to 1.37) [30 participants].
- Another RCT (34 patients) compared autofluorescence-with tetracycline fluorescence-guided sequestrectomy for the surgical treatment of MRONJ finding no difference at one-year follow-up RR = 1.05 (95%CI; 0.86 to 1.30).
- 3 RCTs investigated the effect of growth factors and autologous platelet concentrates on MRONJ healing rates: –
- Platelet-rich fibrin after bone surgery versus surgery alone RR = 1.05 (95%CI; 0.90 to 1.22) [47 patients].
- Bone morphogenetic protein-2 together with platelet- rich fibrin versus platelet-rich fibrin alone RR = 1.10 (95%CI; 0.94 to 1.29) [55 patients].
- Concentrated growth factor and primary wound closure versus primary wound closure only RR = 1.38 (95%CI; 0.81 to 2.34) [28 patients].
- 2RCTs focused on pharmacological treatment with teriparatide: –
- Teriparatide 20 μg daily versus placebo in addition to standard care RR = 0.96 (95%CI; 0.31 to 2.95) [33 patients].
- Teriparatide 56.5 μg weekly versus teriparatide 20 μg daily in addition to standard care RR = 1.60 (95%CI; 0.25 to 1.44) [12 patients].
- There is low or very low certainty evidence on interventions for the prophylaxis or treatment of MRONJ.
Conclusions
The authors concluded: –
Prophylaxis of medication-related osteonecrosis of the jaw
One open-label RCT provided some evidence that dental examinations at three-month intervals and preventive treatments may be more effective than standard care for reducing the incidence of medication-related osteonecrosis of the jaw (MRONJ) in individuals taking intravenous bisphosphonates for advanced cancer. We assessed the certainty of the evidence to be very low.
There is insufficient evidence to either claim or refute a benefit of the interventions tested for prophylaxis of MRONJ in patients with antiresorptive therapy undergoing dentoalveolar surgery. Although some interventions suggested a potential large effect, the studies were underpowered to show statistical significance, and replication of the results in larger studies is pending.
Treatment of medication-related osteonecrosis of the jaw
The available evidence is insufficient to either claim or refute a benefit, in addition to standard care, of any of the interventions studied for the treatment of MRONJ.
Comments
MRONJ is a relatively new clinical problem, so the underlying mechanisms related to the condition are not well known. Consequently, prevention and treatment for the well -known complication of antiresorptive medication remains a challenge. This Cochrane review updates the previous version (Dental Elf – 18th Oct 2017) and includes an additional 8 studies. However all the included studies were at high risk of bias in one or more of the domains assessed so the available evidence is of low or very low certainty. High quality prospective studies are needed to improve the quality of evidence. For prevention the reviewers highlight that large sample sizes are required to detect meaningful effects. For research into future treatments the Cochrane reviewers suggest high quality RCTs assessing surgical versus non-surgical protocols or conservative versus aggressive surgical protocols for the stage-specific treatment of MRONJ. They also note that, the evaluation of add-on effects for adjunct treatments such as HBO, α-tocopherol, pentoxifylline, ozone therapy, or low-level laser therapy, is important. The currently available studies are small so new studies should have meaningful size samples and ensure that outcomes assessors are masked.
Links
Primary Paper
Beth-Tasdogan NH, Mayer B, Hussein H, Zolk O, Peter JU. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst Rev. 2022 Jul 12;7:CD012432. doi: 10.1002/14651858.CD012432.pub3. PMID: 35866376.
Other references
Dental Elf – 18th Oct 2017
Medication-related osteonecrosis of the jaw (MRONJ): treatment and prevention
SDCEP Guidance- Oral Health Management of Patients at risk of MRONJ – March 2017
Dental Elf – Osteonecrosis of the Jaw blogs
Photo Credits
By Coronation Dental Specialty Group – Own work, CC BY-SA 4.0, Link
